APPLICATION NOTE NO. 64
SBE 43 Dissolved Oxygen Sensor --
Background Information, Deployment Recommendations, and Cleaning and Storage
Revised February 2011
![]()
The SBE 43 is a polarographic membrane oxygen sensor having a single output signal of 0 to +5 volts, which is proportional to the temperature-compensated current flow occurring when oxygen is reacted inside the membrane. A Sea-Bird CTD that is equipped with an SBE 43 oxygen sensor records this voltage for later conversion to oxygen concentration using a modified version of the algorithm by Owens and Millard (1985).
The SBE 43 determines dissolved oxygen concentration by counting the number of oxygen molecules per second (flux) that diffuse through the membrane from the ocean environment to the working electrode. At the working electrode (cathode), oxygen gas molecules are converted to hydroxyl ions (OH-) in a series of reaction steps where the electrode supplies four electrons per molecule to complete the reaction. The sensor counts oxygen molecules by measuring the electrons per second (amperes) delivered to the reaction. At the other electrode (anode), silver chloride is formed and silver ions (Ag+) are dissolved into solution. Consequently, the chemistry of the sensor electrolyte changes continuously as oxygen is measured, resulting in a slow but continuous loss of sensitivity that produces a continual, predictable drift in the sensor calibration with time. This electro-chemical drift is accelerated at high oxygen concentrations and falls to zero when no oxygen is being consumed. Accordingly, sensor storage and deployment strategies that produce zero- or near zero-oxygen environments when the sensor is not being sampled can be used to substantially reduce electro-chemical drift, improving long-term data quality.
Membrane fouling also contributes to drift by altering the oxygen diffusion rate through the membrane, thus reducing sensitivity. Non-biological fouling, occurring for example if the SBE 43 was profiled through an oil slick, typically produces an immediate jump toward low oxygen. Biological fouling, particularly on moorings, can be troublesome, because the living organisms either consume or create oxygen. Without protection and/or routine cleaning, a micro-environment around the sensor can produce oxygen levels that are different from the true ambient conditions. By recognizing fouling, both episodic and gradual in nature, and promptly cleaning the sensor using the procedures in this application note, accuracy can be restored.
In 2007, Sea-Bird began shipping SBE 43s with a black plastic plenum in place of the original white plastic plenum, for SBE 43s intended for mooring applications. When our inventory of white plastic plenums is used up, we will begin building all SBE 43s with black plastic plenums. In 2007, Sea-Bird also began shipping SBE 43s intended for mooring applications with black Tygon tubing in place of clear Tygon tubing (SBE 43s intended for profiling applications will continue to be plumbed with clear tubing). The black plenum and tubing minimize light entering the system, and reduce biological fouling.
The concentration of oxygen in the environment can be computed given the flux of oxygen and the geometry of the diffusion path. The permeability of the membrane to oxygen is a function of temperature and ambient pressure and is taken into account in the calibration equation. The algorithm to compute oxygen concentration requires measurements of water temperature, salinity, pressure, and oxygen sensor output voltage. When the oxygen sensor is interfaced with a Sea-Bird CTD, all of these parameters are measured by the CTD system.
The oxygen sensor consumes the oxygen in the water very near the surface of the sensor membrane. If there is not an adequate flow of new water past the membrane, the sensor will give a reading that is lower than the true oxygen concentration. Additionally, if the flow rate is not constant, the sensor response time will vary, causing dynamic error, particularly when profiling. Maximum accuracy requires that water be pumped (across the membrane) at rates from 20 to 40 ml/second, as provided on Sea-Bird CTDs with SBE 5T or 5P pumps.
Temperature differences between the water and oxygen sensor can lead to errors in the oxygen measurement. The SBE 43 minimizes this difference by using materials that equilibrate rapidly with the environment and incorporating a thermistor placed under the membrane, at the cathode, for accurate temperature compensation. As a result, the SBE 43 is less susceptible to error when profiling through areas of high temperature gradients than previous oxygen sensors.
As discussed above, the oxygen sensor consumes the oxygen in the water near the sensor membrane. In moored applications, this requires that water be pumped past the oxygen sensor. When used with a SEACAT (SBE 16, 16plus, 16plus-IM, 16plus V2, 16plus-IM V2, or 19plus or 19plus V2 in moored mode), the SBE 43 flow chamber (plenum) is connected in-line between the pump and conductivity sensor. The pump does not run between samples, trapping water in the plenum. Because the sensor is continuously polarized by an internal battery, oxygen continues to be consumed between samples. The sensor depletes oxygen in the water close to the membrane. If you were to observe the sensor output after the pump stopped, the oxygen concentration inside the plenum would approach a steady state well below ambient oxygen levels. When the pump switches on at the beginning of the next sampling interval, you would observe a curve similar to those shown below for a 0.5-mil membrane. The water flow establishes a normal boundary layer at the membrane, and the sensor equilibrates to the ambient oxygen level. Time required to reach 99% of the final equilibrium value depends on temperature (faster equilibration in warmer water) and on the sensor membrane thickness (faster equilibration with a thinner membrane).
Vertical arrows on the plot show the point at which the sensor has achieved 99% of the final value at each temperature.
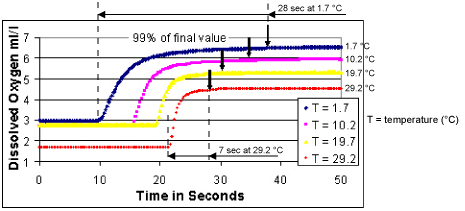
Response Time at Several Temperatures for a 0.5-mil Membrane
Prior to 2007, all SBE 43s were sold with a 0.5-mil thick membrane. Beginning in 2007, Sea-Bird began offering two membrane thicknesses – 0.5 mil (faster response, typically for profiling applications) and 1.0 mil (slower response but more durable, typically for moored applications).
Summary of Response Times for Moored Applications
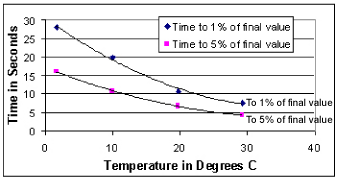
The first plot below is derived from the preceding plot and may be used to determine the time required from power-up and pump turn-on to the availability of an acceptable dissolved oxygen sample with a 0.5-mil membrane. For simplicity, we generally recommend a minimum pump time of 15 seconds for 15 °C and warmer water, and reference the 1% curve below for colder water.
Summary of Response Times for 0.5-mil Membrane
Example -- SBE 16plus
with SBE 43 with 0.5-mil membrane:
If working in 20 °C water and wanting oxygen data to
within 1% of actual oxygen concentrations, pump time of at least 11 seconds is
required. Set the SBE 16plus to pump during the entire sample (PumpMode=2) *,
and set the delay before sampling to 15 seconds (DelayBeforeSampling=15) *.
We have allowed 4 extra seconds of pump time; this ensures that the sample
will still be good if the SBE 16plus is in colder than expected water. Note that
longer pump times reduce battery endurance.
* See the appropriate CTD manual for the exact format of these commands;
they vary, depending on the product and the telemetry interface.
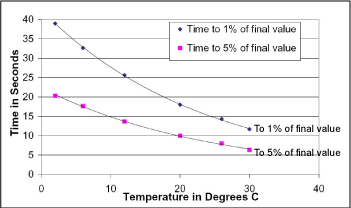
The next plot was derived in a similar fashion, and may be used to determine the time required from power-up and pump turn-on to the availability of an acceptable dissolved oxygen sample with a 1.0-mil membrane. For simplicity, we generally recommend a minimum pump time of 25 seconds for 15 °C and warmer water, and reference the 1% curve below for colder water.
Summary of Response Times for 1.0-mil
Membrane
SBE 43 oxygen sensors can be used for hours in hydrogen sulfide rich environments with no ill effects to sensor elements or signal calibration.
Poisoning of oxygen sensors in hydrogen sulfide environments was a phenomenon common to early sensor designs that used silver as the cathode element. The SBE 43 uses a noble metal (gold) as the cathode and silver as the anode, and shows no degradation of signal or calibration when used for profiling in hydrogen sulfide environments. In particular, a month of intensive hydrographic profiles in the Black Sea using Sea-Bird oxygen sensors has demonstrated that these sensors can operate in the H2S rich depths for durations of hours without any degradation of signal or calibration over that experienced in equivalent profiling work in the open, oxygenated ocean.
We have no laboratory or field evidence of the effect of mooring Sea-Bird oxygen sensors in H2S rich environments for periods of days to months.
Sea-Bird’s algorithm has the following form:
Oxygen (ml/l) = [Soc * (V + Voffset + tau(T,P) * dV/dt)] * Oxsol (T,S) * (1.0 + A*T + B*T2 + C*T3) * e (E*P/K)
where.....
| Description | Symbol | Definition |
| Computed | Oxygen | Dissolved oxygen concentration (ml/l) |
| Measured Parameters | T | CTD Temperature (ºC) |
| P | CTD Pressure (decibars) | |
| S | CTD Salinity (PSU) | |
| V | SBE 43 temperature-compensated output oxygen signal (volts) | |
| Calibration Coefficients | Soc | Oxygen signal slope |
| Voffset | Voltage at zero oxygen signal | |
| A, B, C | Residual temperature correction factors | |
| E | Pressure correction factor | |
| tau20 | Sensor time constant tau(T,P) at 20 ºC, 1 atmosphere, 0 PSU; slope term in calculation of tau(T,P) | |
| D1, D2 | Temperature and pressure correction factors in calculation of tau(T,P) | |
| H1, H2, H3 | Hysteresis correction factors | |
| Calculated Value | Oxsol (T,S) | Oxygen saturation value after Garcia and Gordon (1992); see Appendix A |
| dV/dt | Time derivative of SBE 43 output oxygen signal (volts/second) | |
| tau(T,P) |
Sensor time constant at temperature and
pressure = tau20 * exp (D1 * P + D2 * [T - 20]) |
|
| K | Absolute temperature | |
Our software requires you to enter Soc, Voffset, A, B, C, E, tau20, D1, D2, H1, H2, and H3 in the configuration (.con or .xmlcon) file; values are taken from the Calibration Sheet provided with the sensor.
Note: H1, H2, and H3 values are available on calibration sheets for SBE 43s calibrated after September 2008. See Application Note 64-3: SBE 43 Dissolved Oxygen Sensor Hysteresis Corrections for the appropriate values to use if your calibration sheet does not show these coefficients.
Tau Correction
Note the derivative term [tau(T,P) * dV/dt]; this correction term’s function is to improve the response of the measured signal in regions of large oxygen gradients. However, this term also amplifies residual noise in the signal (especially in deep water), and in some situations this negative consequence overshadows the gains in signal responsiveness. In Seasave V7 and SBE Data Processing version 7.18b or later, this Tau correction can be disabled if desired, deleting the entire derivative term from the equation for calculated oxygen.
Hysteresis Correction
Under extreme pressure, changes can occur in gas permeable Teflon membranes that affect their permeability characteristics. Some of these changes (plasticization and amorphous/crystalinity ratios) have long time constants and depend on the sensor’s time-pressure history. These slow processes result in hysteresis in long, deep casts. The hysteresis correction algorithm (using H1, H2, and H3 values entered for the SBE 43 in the configuration [.con or .xmlcon] file) operates through the entire data profile and corrects the oxygen voltage values for changes in membrane permeability as pressure varies. At each measurement, the correction to the membrane permeability is calculated based on the current pressure and how long the sensor spent at previous pressures.
Hysteresis responses of membranes on individual SBE 43 sensors are very similar, and in most cases the default hysteresis parameters provide the accuracy specification of within 2% of true value. For users requiring higher accuracy (±1 µmol/kg), the parameters can be fine-tuned, if a complete profile (descent and ascent) made preferably to greater than 3000 meters is available. H1, the effect’s amplitude, has a default of -0.033, but can range from -0.02 to -0.05 between sensors. H2, the effect’s non-linear component, has a default of 5000, and is a second-order parameter that does not require tuning between sensors. H3, the effect’s time constant, has a default of 1450 seconds, but can range from 1200 to 2000. Hysteresis can be eliminated by alternately adjusting H1 and H3 in the configuration file during analysis of the complete profile. Once established, these parameters should be stable, and can be used without adjustment on other casts with the same SBE 43.
The following Sea-Bird software allows you to select the SBE 43 oxygen sensor (labeled Oxygen, SBE) and use the Sea-Bird equation documented in this application note when setting up the configuration file for the CTD:
The latest version of the software is available for download from our website.
Note:
There are several types of oxygen data that can be calculated, as desired, in all of these software versions:
Data Conversions
Sea-Bird uses the following equations to convert oxygen to various engineering units:
[mg/L] = [ml/L] * 1.42903
[micromole/Kg] = [ml/L] * 44660 / (sigma_theta(P,T,S) + 1000)
For the micromole/Kg conversion, there is disagreement in the scientific community about the conversion constant 44660 should be used:
The value 44660 is exact for oxygen gas.
The value 44615 is the average value for atmospheric gas (N2,O2,Ar,H2O,CO2,...). It is not exact for any individual gas, but has been used historically by oceanographers.
The argument distills to exact versus historic, with oceanographers split; Sea-Bird uses 44660 in all software calculations.
Sea-Bird has altered our recommendations with regard to cleaning and storage of the SBE 43. In the past, we recommended using Triton X-100 detergent for the combined purpose of degreasing and discouraging biological growth. We recently discovered that prolonged exposure of Triton X-100 to the sensor membrane is harmful and causes the sensor’s calibration to drift. Our present recommendation, detailed below, is to continue to use Triton X-100 for degreasing (with a short wash), then use a short wash with a dilute bleach solution to reduce biological growth, and store the sensor in an anoxic (or near zero oxygen) condition. See Materials below for a discussion of Triton X-100, bleach, and water.
Avoid fouling the oxygen membrane with oil or grease as this causes a calibration shift toward erroneously low readings. An oil-fouled membrane can be cleaned using the following procedures.
CAUTION: During service and storage, maintain temperature at or below 30 ºC (86 ºF). If temperatures are raised above 40 ºC (104 ºF), sensors exhibit a temporary increase in sensitivity of a few percent. This relaxes back to historical sensitivity after a few days when temperatures return below 30 ºC (86 ºF).
- Flush the sensor for 1 minute with a 1% solution of Triton X-100 warmed to 30 ºC (86 ºF). Drain and flush with warm (not hot) fresh water for 5 minutes.
- Soak the sensor for 1 minute in a 500 - 1000 ppm solution of Bleach. After the soak, drain and flush with warm (not hot) fresh water for 5 minutes.
- If there is no danger of freezing, loop tubing from inlet to outlet. Place a small piece of clean sponge, slightly dampened with fresh, clean water, in the center of the tubing (not near the membrane).
- If there is danger of freezing, shake all excess water out of the plenum and loop tubing from inlet to outlet, leaving the sensor membrane dry.
- Because the sensor is continuously polarized by a internal battery, oxygen in the plenum and tubing will continue to be consumed, depleting the electrolyte and causing drift. Storing the sensor in a zero-oxygen environment will stop calibration drift between uses. To minimize drift during storage, if possible, connect one end of the tubing loop to the plenum, displace the air in the plenum and tubing with Nitrogen gas, and connect the other end of the tubing to the plenum. If tubing is not available, displace the air in the plenum with Nitrogen gas and close off the plenum with a cap on each end (tape can be used if nothing else is available); do not insert a cap or plug inside the plenum.
Materials
Notes:
Ts = ln [(298.15 – T) / (273.15 + T)]
Oxsol(T,S) = exp {A0 +
A1(Ts) + A2(Ts)2 + A3(Ts)3 + A4(Ts)4 + A5(Ts)5
+
S * [B0 + B1(Ts) + B2(Ts)2
+ B3(Ts)3] + C0(S)2}
where
The table below contains oxygen saturation values at atmospheric pressure calculated using the Oxsol equation. Units are ml/l. To compute mg/l, multiply the values in the table by 1.42903.
| Oxsol: Oxygen Saturation Concentrations in Fresh and Ocean Water (ml/l) | |||||||||
|
Temperature (°C) |
Salinity (PSU) | ||||||||
| 0 | 5 | 10 | 15 | 20 | 25 | 30 | 32 | 35 | |
| -2 | 10.84 | 10.46 | 10.10 | 9.74 | 9.40 | 9.07 | 8.75 | 8.63 | 8.45 |
| 0 | 10.23 | 9.88 | 9.54 | 9.21 | 8.90 | 8.59 | 8.30 | 8.18 | 8.01 |
| 2 | 9.68 | 9.35 | 9.04 | 8.73 | 8.44 | 8.15 | 7.88 | 7.77 | 7.61 |
| 4 | 9.17 | 8.87 | 8.58 | 8.29 | 8.02 | 7.75 | 7.49 | 7.39 | 7.24 |
| 6 | 8.71 | 8.43 | 8.15 | 7.89 | 7.63 | 7.38 | 7.14 | 7.05 | 6.91 |
| 8 | 8.29 | 8.02 | 7.77 | 7.52 | 7.28 | 7.04 | 6.82 | 6.73 | 6.60 |
| 10 | 7.90 | 7.65 | 7.41 | 7.18 | 6.95 | 6.73 | 6.52 | 6.44 | 6.31 |
| 12 | 7.54 | 7.31 | 7.08 | 6.86 | 6.65 | 6.45 | 6.25 | 6.17 | 6.05 |
| 14 | 7.21 | 6.99 | 6.78 | 6.57 | 6.37 | 6.18 | 5.99 | 5.92 | 5.81 |
| 16 | 6.91 | 6.70 | 6.50 | 6.31 | 6.12 | 5.93 | 5.75 | 5.68 | 5.58 |
| 18 | 6.62 | 6.43 | 6.24 | 6.06 | 5.88 | 5.70 | 5.53 | 5.47 | 5.37 |
| 20 | 6.36 | 6.18 | 6.00 | 5.82 | 5.65 | 5.49 | 5.33 | 5.27 | 5.17 |
| 22 | 6.12 | 5.94 | 5.77 | 5.61 | 5.45 | 5.29 | 5.14 | 5.08 | 4.99 |
| 24 | 5.89 | 5.72 | 5.56 | 5.41 | 5.25 | 5.10 | 4.96 | 4.90 | 4.82 |
| 26 | 5.68 | 5.52 | 5.37 | 5.22 | 5.07 | 4.93 | 4.79 | 4.74 | 4.66 |
| 28 | 5.48 | 5.33 | 5.18 | 5.04 | 4.90 | 4.77 | 4.63 | 4.58 | 4.51 |
| 30 | 5.29 | 5.15 | 5.01 | 4.87 | 4.74 | 4.61 | 4.49 | 4.44 | 4.36 |
| 32 | 5.11 | 4.98 | 4.84 | 4.71 | 4.59 | 4.46 | 4.34 | 4.30 | 4.23 |
Note: As implemented in Sea-Bird software, the Oxsol equation is valid for -5 < T < 50 and 0 < S < 60. Outside of those ranges, the software returns a value of -99 for Oxsol.
Carritt, D.E. and J.H. Carpenter. 1966: Comparison and evaluation of currently employed modifications of the Winkler method for determining dissolved oxygen in seawater. J. Mar. Res. 24(3), 286-318.
Clesceri, L.S. A.E. Greenberg, and R.R. Trussell ed. 1989, Standard methods for the examination of water and wastewater, 17th edition, American Public Health Assoc. Washington D.C. ISBN 0-87553-161-X.
Gnaigner, E., and H. Forstner, Ed., 1983: Polarographic Oxygen Sensors: Aquatic and Physiological Applications, Springer-Verlag, 370 pp.
Millard, R, C., Jr., 1982: CTD calibration and data processing techniques at WHOI using the 1978 practical salinity scale. Proc. Int. STD Conference and Workshop, La Jolla, Mar. Tech. Soc., 19 pp.
Owens, W.B., and R.C. Millard Jr., 1985: A new algorithm for CTD oxygen calibration. J. Physical Oceanography, 15, 621-631.
Garcia and Gordon (1992) "Oxygen solubility in seawater: Better fitting equations", Limnology & Oceanography, vol 37(6), p1307-1312.
Application Note 64-1: Plumbing Installation -- SBE 43 DO Sensor and Pump on a CTD
Application Note 64-2: SBE 43 Dissolved Oxygen Sensor Calibration and Data Corrections using Winkler Titrations
Application Note 64-3: SBE 43 Dissolved Oxygen Sensor -- Hysteresis Corrections
| Date | Description |
| - Initial release. | |
| September 2002 | - Modify language and equation
consistent with Application Note 64-2. - Modify cleaning recommendations (caution about not putting triton directly on membrane). |
| January 2004 | - Correct equation at beginning of Appendix A, which was missing a bracket. |
| December 2004 | - Change cleaning recommendations: short (1 minute) soak in dilute bleach solution and short (1 minute) soak in dilute Triton solution. |
| May 2004 | - Add information on use in hydrogen sulfide rich environments. |
| August 2005 | - Add information on “oxygen, SBE” vs. “oxygen saturation” vs “percent saturation” in software. |
| November 2005 | - Add information on use in moored mode – discuss equilibration time vs temperature, and pump time. |
| December 2005 | - Add information on Nitrogen gas in Tygon tubing for storage. |
| October 2006 | - Update name of manufacturer and web link for Triton. |
| February 2007 |
- Update temperature for Triton solution
cleaning to 30 °C (was 40 °C). - Add caution about storing at temperatures above 30 °C. |
| July 2007 |
- Add response time curve for new 1 mil
membrane, provide DelayBeforeSampling= recommendation of 25 seconds. - Change title of application note to reflect what is covered. - Discuss black plenum and black tubing. - Add information about SBE 5P pump. - Update for Seasave V7. - Software (Seasave V7, Seasave Win32, and SBE Data Processing) was updated to accommodate new DO equation, mention new equation to be released in Fall 2007. |
| April 2008 |
- Introduce Sea-Bird equation, update
equations, etc. - Update Appendix A (provide Oxsol values instead of Oxsat values). |
| November 2008 | - Update to correspond to software changes in SBE Data Processing and Seasave V7 versions 7.18b, providing information on tau and hysteresis corrections. |
| February 2010 |
- SBE Data Processing and Seasave V7
version 7.20b: modification to Oxsol equation (Garcia & Gordon only)
to extend ranges to (-5 < t < 50, 0 < s < 60) instead of (-2 < t <
40, 0 < s < 42). Outside those ranges, it returns a value of -99.0. - Correct documentation of A4 in Garcia & Gordon Oxsol, should be negative (was listed as positive). - Add information on .xmlcon configuration file. - Update address. |
| February 2011 | - Add reference to Application Note 64-3 for information on hysteresis coefficients H1, H2, and H3. |
![]()
Last modified: 15 Feb 2011
Sea-Bird Home Phone: (+1) 425-643-9866 Fax: (+1) 425-643-9954 E-mail: seabird@seabird.com